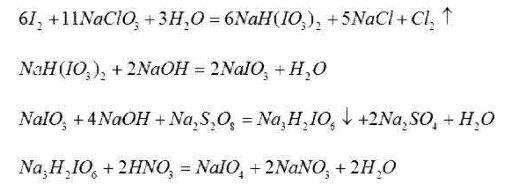What is Sodium Periodate?
Sodium periodate is a colorless or white tetragonal crystalline powder. It is the primary raw material of the pharmaceutical industry, used in the food and pharmaceutical industries. It can be used as an oxidizing agent, general analysis reagent and chromatographic analysis reagent, pharmaceutical intermediate, and health care product.
The preparation method one of sodium periodate
Usually, the production method of sodium periodate is chlorine gas oxidation.
It is divided into two steps: the preparation of trihydrogen trisodium periodate and the preparation of sodium periodate. It is that iodine is added to the sodium hydroxide solution, and chlorine gas is introduced to react to generate trisodium dihydrogen periodate (Na3H2IO6) and sodium chloride solution. After separating the impurities, add trisodium dihydrogen periodate into the nitric acid solution to react to form a mixed solution of sodium periodate and sodium nitrate. Using the poor solubility of sodium periodate and sodium nitrate, crystallization, separation, concentration, and precipitation of sodium periodate. Then go through the centrifugal dehydration step and drying step to make sodium periodate finished product. Trisodium dihydrogen periodate is a white crystalline powder, easily soluble in dilute nitric acid and concentrated sodium hydroxide solution, and very slightly soluble in water.
The reaction equation of the chlorine oxidation method is:
The disadvantages of this method are: the consumption of raw materials is large, and the economic cost is high. From the reaction equation, the production of 1 mol of sodium periodate requires the consumption of 10 mol of sodium hydroxide, 3.5 mol of chlorine, and the production of 7 mol of sodium chloride. This has resulted in the discharge of a large amount of stock solution and high-concentration sodium chloride solution, causing great environmental pollution and increasing the difficulty of safe and environmentally friendly production. At the same time, a large amount of chlorine gas will be used in the preparation process, which is a yellow-green poisonous gas with a strong pungent smell. Therefore prepare sodium periodate with the chlorine gas oxidation method, also very strict to the requirement of safety production, method is loaded down with trivial details and is complicated.
The preparation method two of sodium periodate
The preparation step of dihydro trisodium periodate, the preparation step of sodium periodate, the crystallization separation step, the centrifugal dehydration step, and the drying step.
It is characterized in that: the preparation steps of the described sodium dihydrogen trisodium periodate include the following production steps in sequence:
(1) Preparation steps of sodium hydrogen iodate: Mix sodium chlorate, iodine, and water, and adjust the mixed solution with concentrated nitric acid to make the pH value = 1. Wherein the molar ratio of sodium chlorate, iodine, and water is 11: 6: 3, the solution is heated for 0.5-1 hour to react, and the chlorine gas generated is absorbed with lye to obtain a mixed solution of sodium hydrogen iodate and sodium chloride.
(2) The preparation step of sodium iodate: adding sodium hydroxide solution to the mixed solution of sodium hydrogen iodate and sodium chloride to react, measuring the pH value of the solution after the reaction, and controlling its pH>7. If ph<7, then add an appropriate amount of sodium hydroxide solution to react until PH>7 to make the solution react completely. Then the solution after the reaction is cooled to 40 DEG C, and the sodium iodate crystals are separated out, and the sodium iodate crystals are centrifugally dehydrated to obtain sodium iodate.
(3) Sodium persulfate oxidation step: adding sodium iodate and sodium persulfate into sodium hydroxide solution and heating to 80-90°C for reaction. Water equivalent to half the volume of the solution before the reaction was added to the solution after the reaction, and the temperature was lowered to stand for stratification for 10-15 minutes to obtain a precipitate of trisodium dihydrogen periodate.
(4) The prepared sodium dihydrogen trisodium periodate is added into the nitric acid solution to react to generate a mixed solution of sodium periodate and sodium nitrate. Utilizing the solubility difference between sodium periodate and sodium nitrate, crystallization, separation, concentration and precipitation of sodium periodate. Then go through centrifugal dehydration step and drying step to make sodium periodate finished product. The crystallization separation step, centrifugal dehydration step and drying step described therein are all conventionally used methods in chemical production.
The chemical equation for the reaction is:
The principle of the chemical reaction is
Iodine and sodium chlorate react under certain conditions to form sodium hydrogen iodate. Sodium hydrogen iodate is then neutralized with sodium hydroxide to obtain sodium iodate. Under alkaline conditions, sodium iodate and sodium persulfate undergo a redox reaction to form a precipitate of trisodium dihydrogen periodate. Dissolve dihydro trisodium periodate precipitate in nitric acid, separate by evaporation, crystallization, centrifugal dehydration, and dry to obtain sodium periodate product.
In the end
Compared with the first method, the second solution has a stable reaction, easy operation process control, safe production, small consumption of raw materials, less waste liquid discharge, and is beneficial to environmental protection. In the process of manufacturing sodium periodate in Jinbangch, try to ensure no pollutant discharge, and minimize the impact on the environment. While conducting chemical production and manufacturing, we do not forget our responsibility to protect the environment. Now, you can contact us to get free samples of Sodium Periodate.